Evaluation of GSFA on Simulated Count Data
-- Multiple Markers, Multiple Factors
Yifan Zhou (zhouyf@uchicago.edu)
2021-05-15
1 Simulation Settings
We simulated our data in a bottom-up fashion: \[G_{im} \overset{i.i.d.}{\sim} \text{Bern}(0.2),\hspace{3mm} \Phi_{ik} \overset{i.i.d.}{\sim} N(0, 1) \Rightarrow Z = G \beta + \Phi\] \[ F_{jk} \sim \text{Bern}(\pi_j),\hspace{3mm} U_{jk} \sim N(0, \sigma_w^2) \Rightarrow W = F \odot U\] \[E_{ij} \sim N(0,\psi_j), Z, W \Rightarrow Y = ZW^T+E\] \(G \in \mathbb{R}^{N \times M}, \beta \in \mathbb{R}^{M \times K}, Z \in \mathbb{R}^{N \times K}, W \in \mathbb{R}^{P \times K}, Y \in \mathbb{R}^{N \times P}.\)
We further generated count data \(C\) from a Poisson distribution based on our simulated continuous gene expression levels \(Y\): \[C_{ij} \sim \text{Pois}(L \text{ exp}(\mu+Y_{ij}))\] with \(L=50000, \mu=\text{log}(1/L)\), which ensures an average read count of 1. Note that, because of the residual errors, the resulting count data are over-dispersed with respect to a Poisson distribution.
For simulation cases in this report,
Sample size \(N = 400\), gene number \(P = 500\), factor number \(K = 10\), and guide/marker number \(M = 6\);
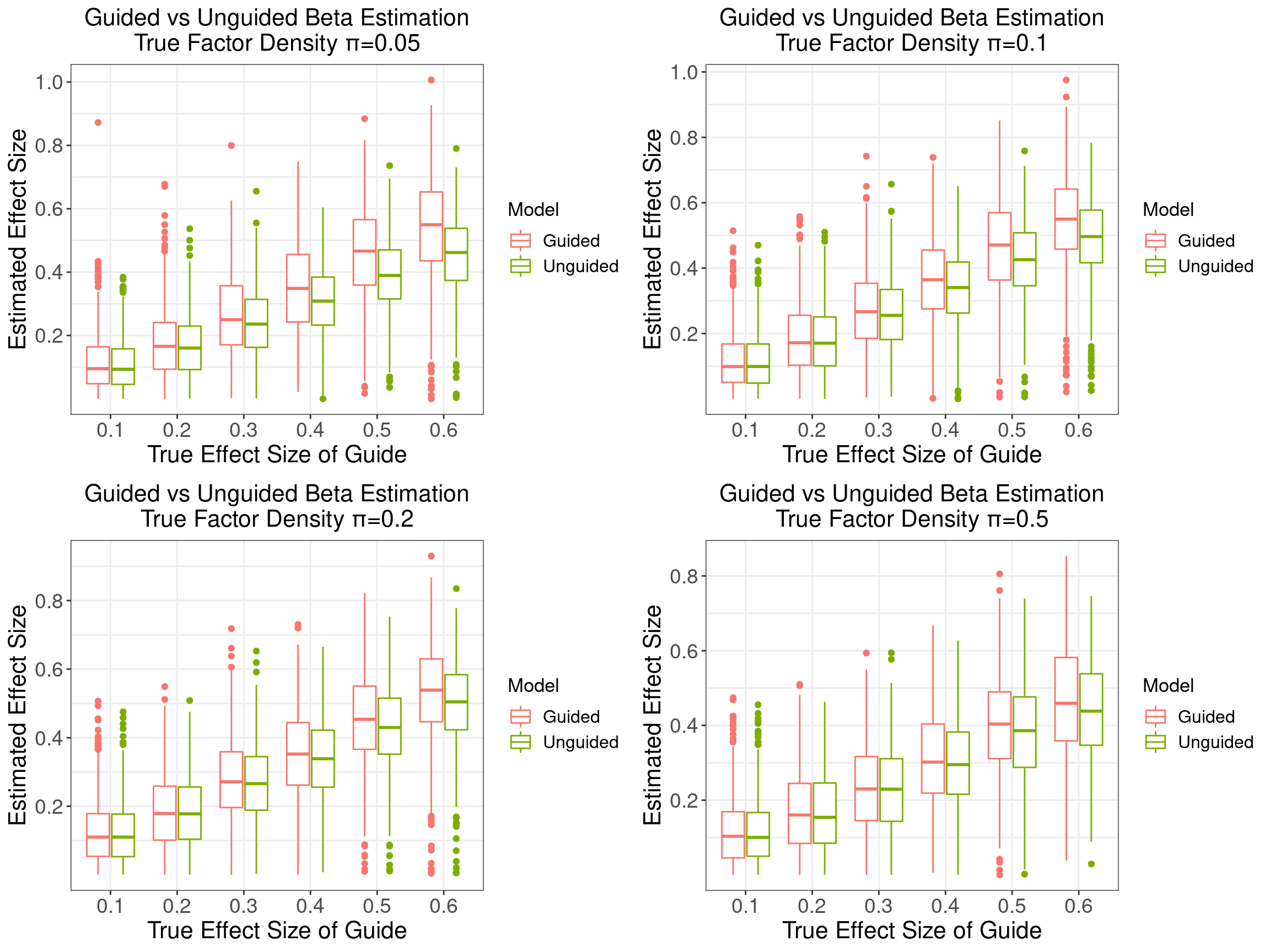
\(\sigma_w^2 = 0.5\), matrix \(\beta\) takes the following form:
The first 6 factors are each associated with a guide, with the effect sizes varying from 0.1 to 0.6, while the last 4 factors are not associated with any guides.
We explored 4 cases when the density parameter \(\pi_j\) is the same across \(j\) but takes a value from 0.1, 0.2, 0.5 to 0.8.
Under each scenario, 500 random datasets were simulated, and both guided and unguided GSFA models were performed on each dataset for 1000 iterations starting from SVD initialization; posterior means were averaged over the last 500 iterations.
2 Estimation of Effect Sizes (\(\beta\)s)
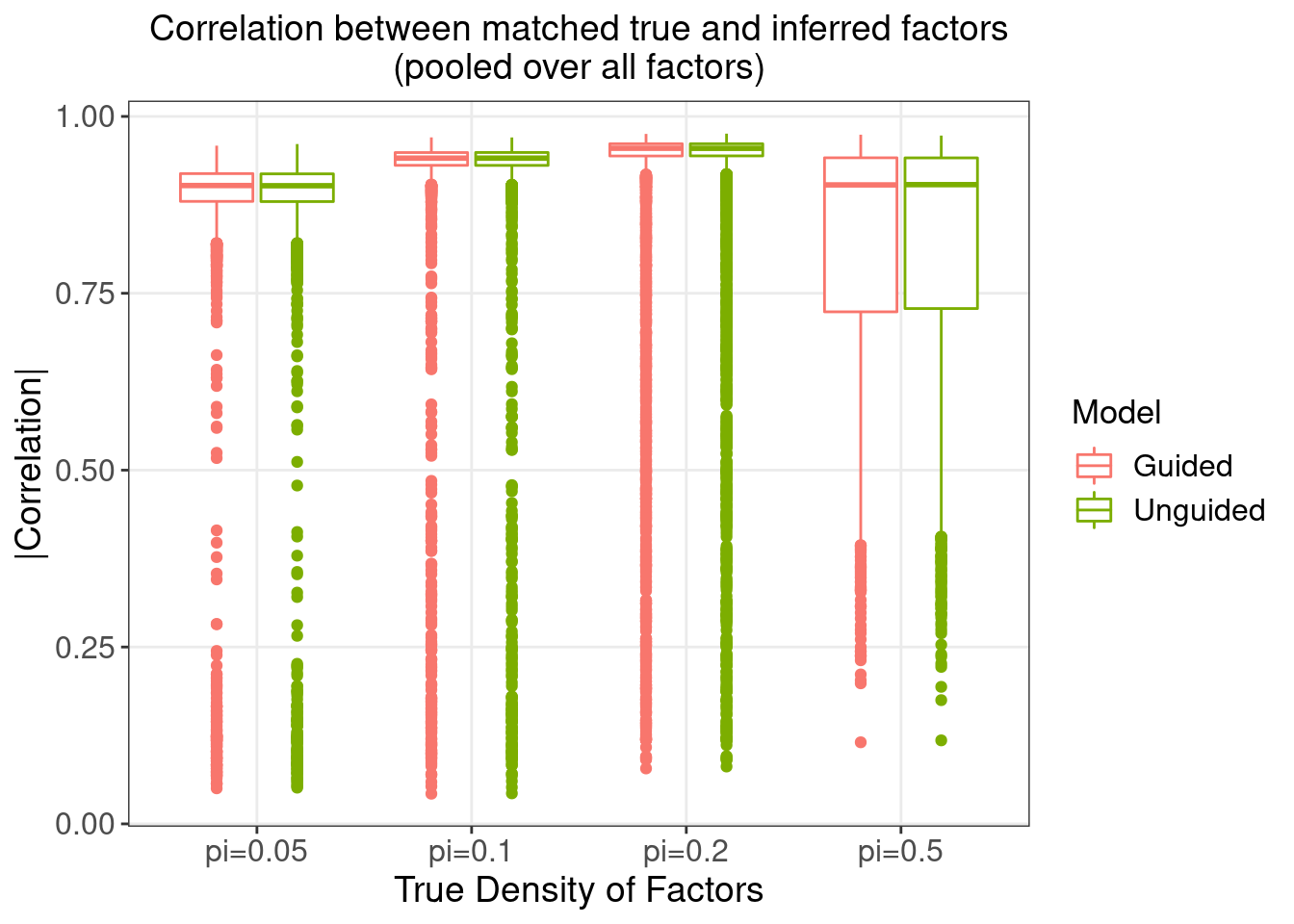
3 Estimation of Factors (\(Z\)s)
We would like to evaluate how different our estimation of the factor matrix, \(\hat{Z}\), is from the true value \(Z\).
3.1 Correlation between matching \(Z\) and \(\hat{Z}\)
On the other hand, since we know the true \(Z\)'s, we can match each of other inferred factors \(\hat{Z}\)'s with the true \(Z\)'s by maximum absolute Pearson correlation.
4 Gene Detection
4.1 Detection methods
- GSFA + Local False Sign Rate (LFSR)
In terms of our GSFA method, we can estimate the effect of a guide/marker on each gene by summarizing over all the factors through the local false sign rate (LFSR):
For gene \(j\) and guide \(m\), based on the posteriors of \(\beta\) and \(W\) of an inference, \[\text{LFSR}_{mj} = \text{min} \Big\{\text{Pr}(\sum_{k=1}^K \beta_{mk}W_{jk} \geq 0 \text{ | Data}), \text{Pr}(\sum_{k=1}^K \beta_{mk}W_{jk} \leq 0 \text{ | Data}) \Big\}\]
- Welch's t-test + FDR
Alternatively, we can simply test each gene directly, measuring whether there is an difference in \(Y_j\) between the two groups of samples under guide \(m\) using Welch's t-test.
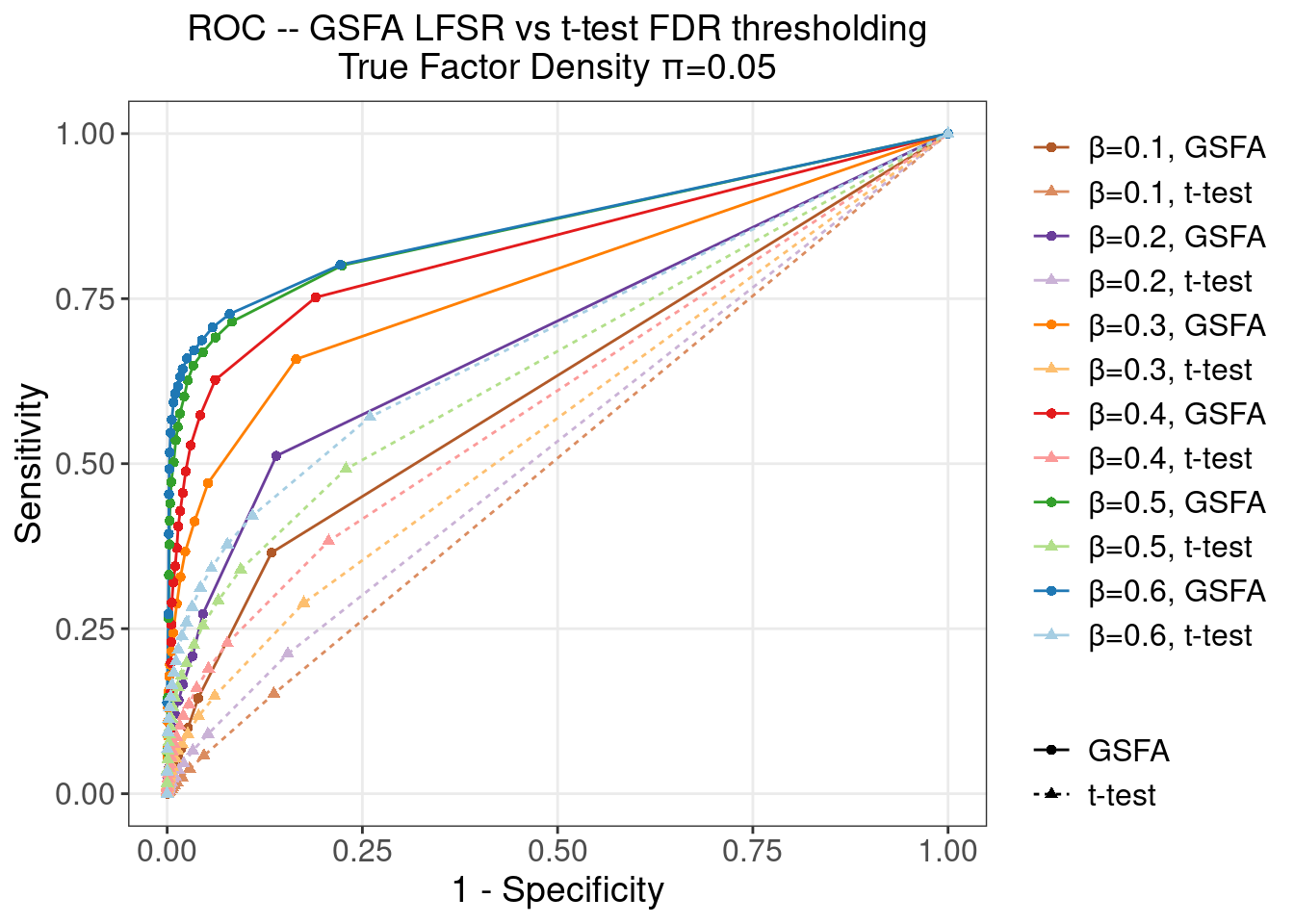
4.2 ROC
To obtain the following ROC curves, we varied the cutoff from 0 to 1 on both LFSR and t-test FDR values.
The actual cutoff values used are (correspond to the datapoints on an ROC curve from left to right)
0, 0.01, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, 0.65, 0.7, 0.75, 0.8, 0.9, 1
4.2.1 \(\pi\) = 0.05
4.2.2 \(\pi\) = 0.1
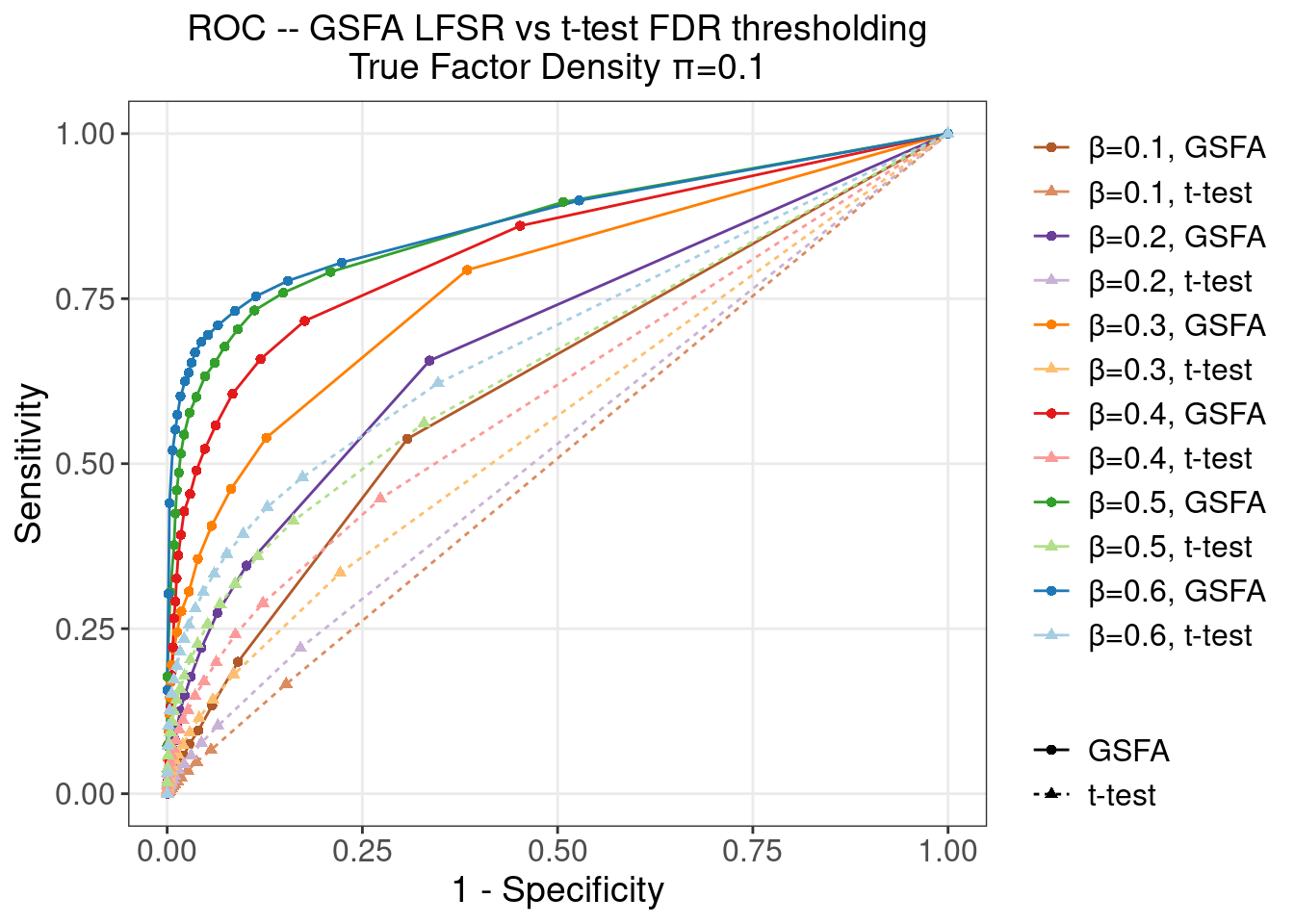
4.2.3 \(\pi\) = 0.2
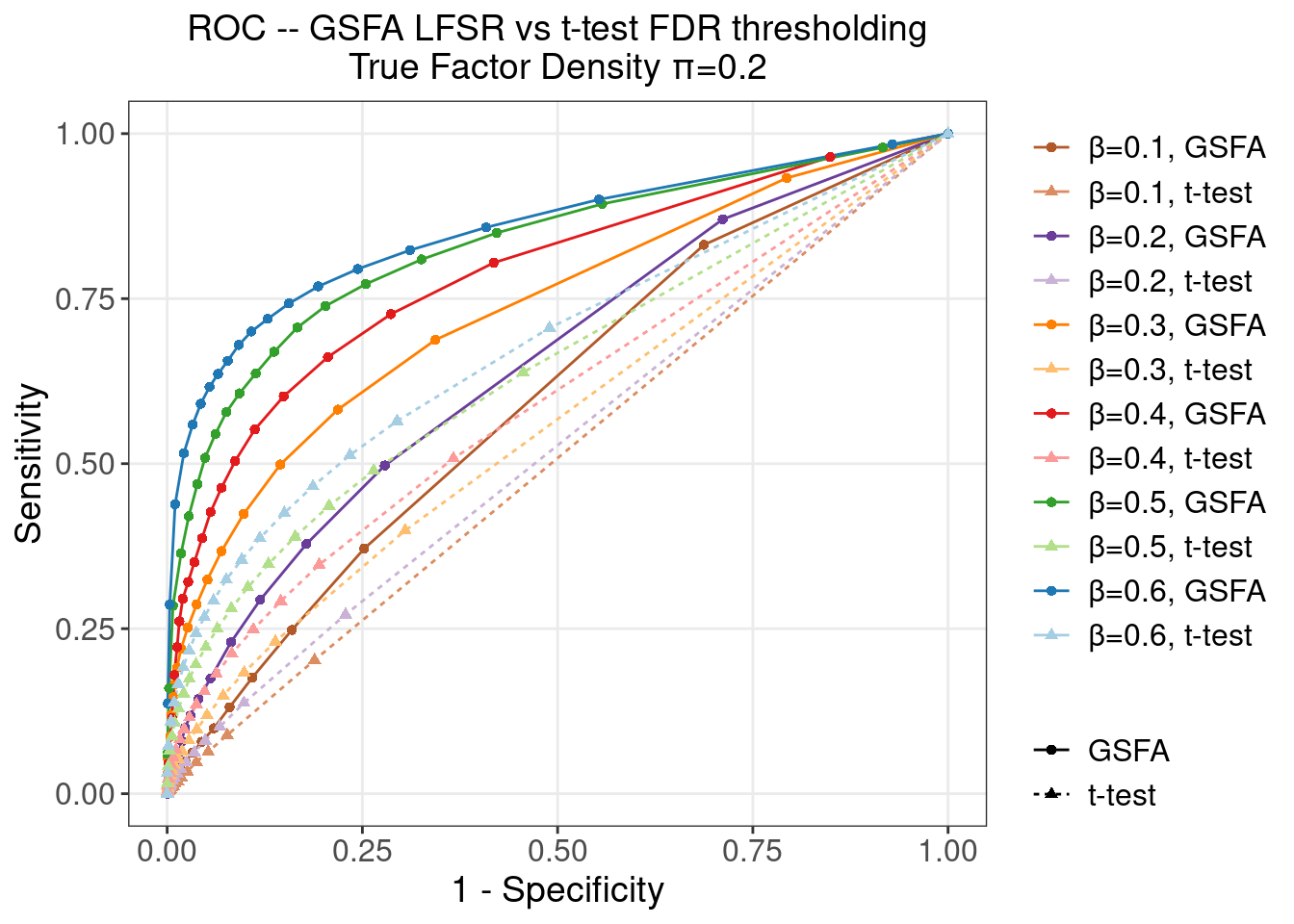
4.2.4 \(\pi\) = 0.5
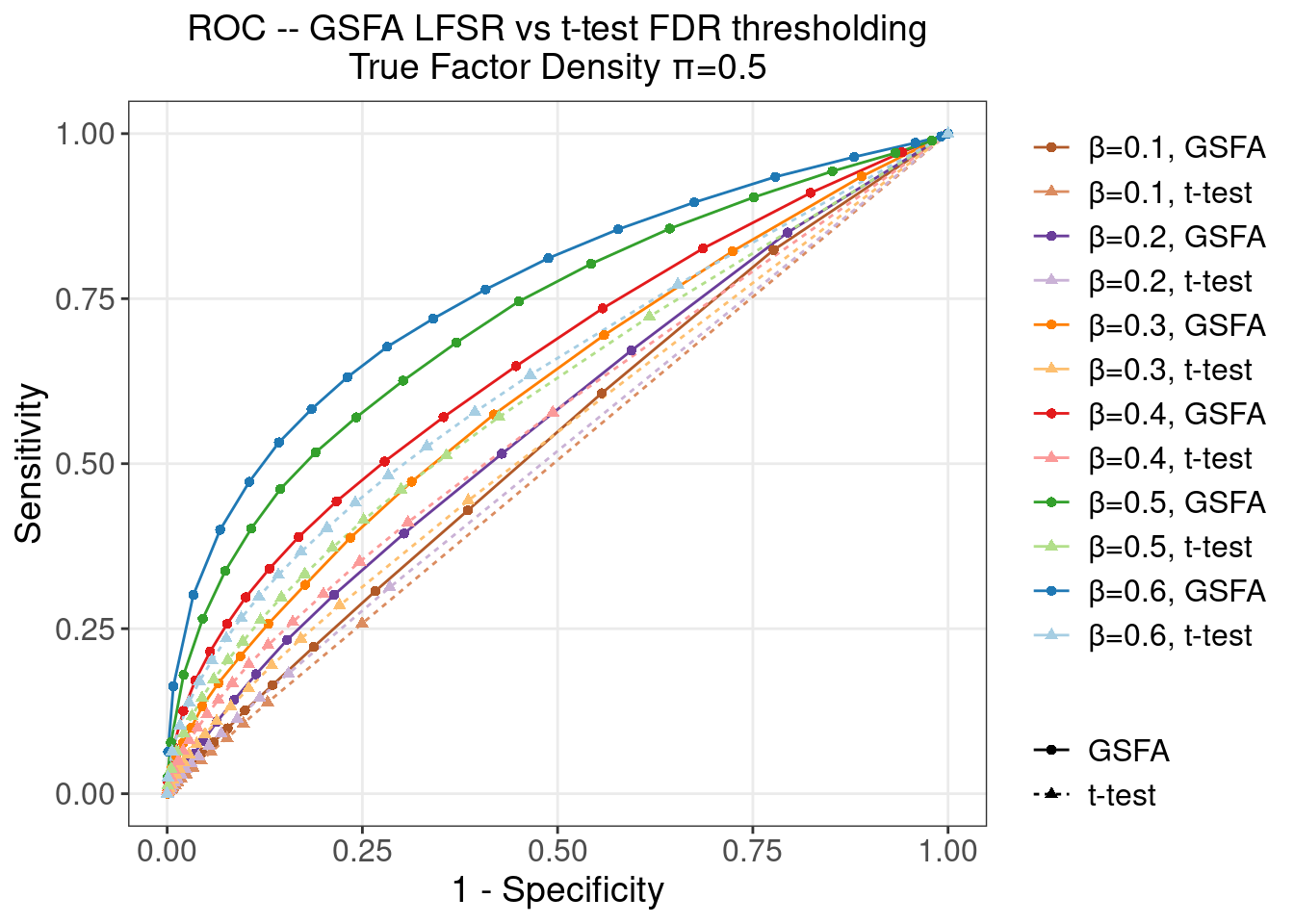
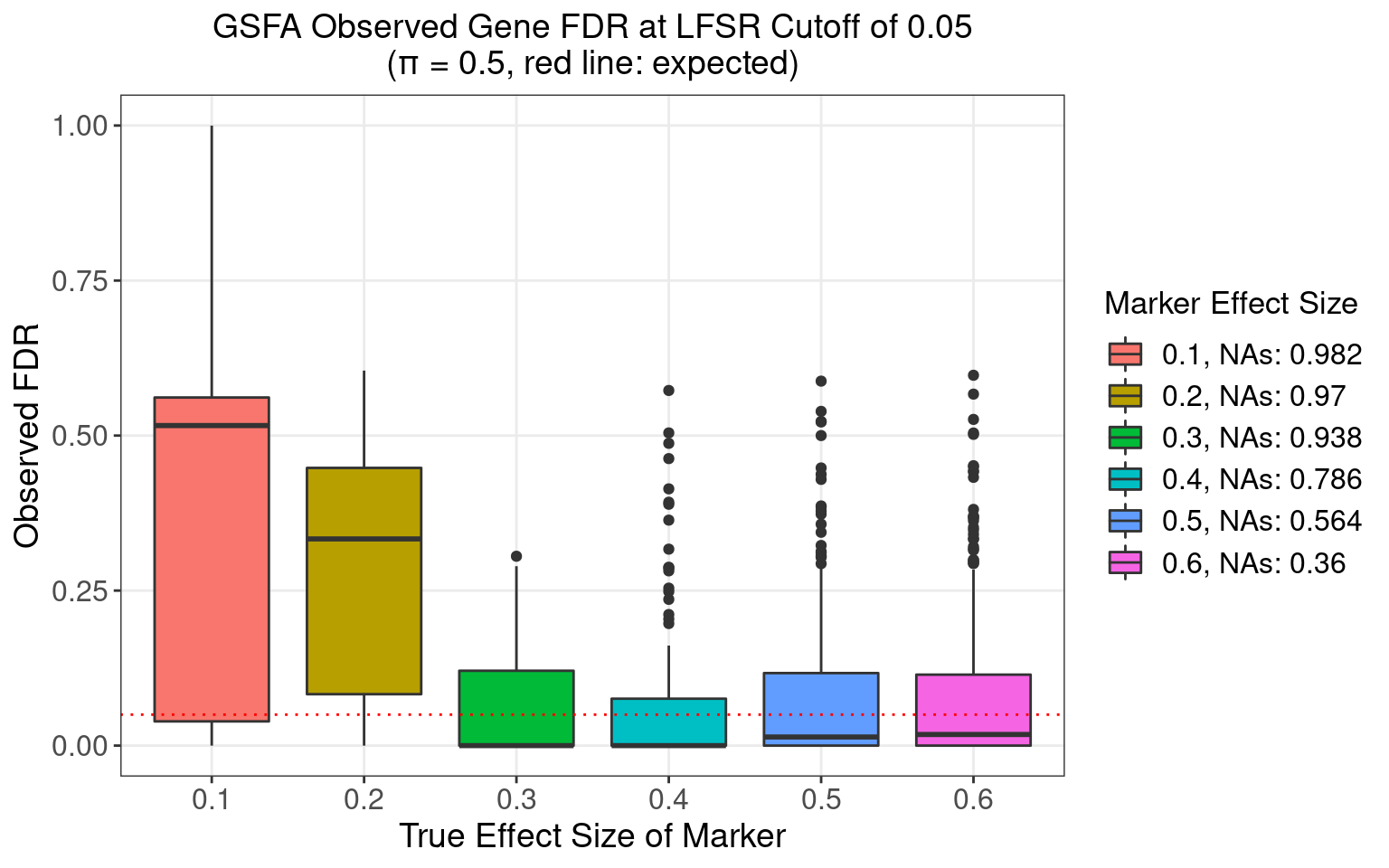
4.3 FDR Calibration at LFSR 0.05
4.3.1 Pooled
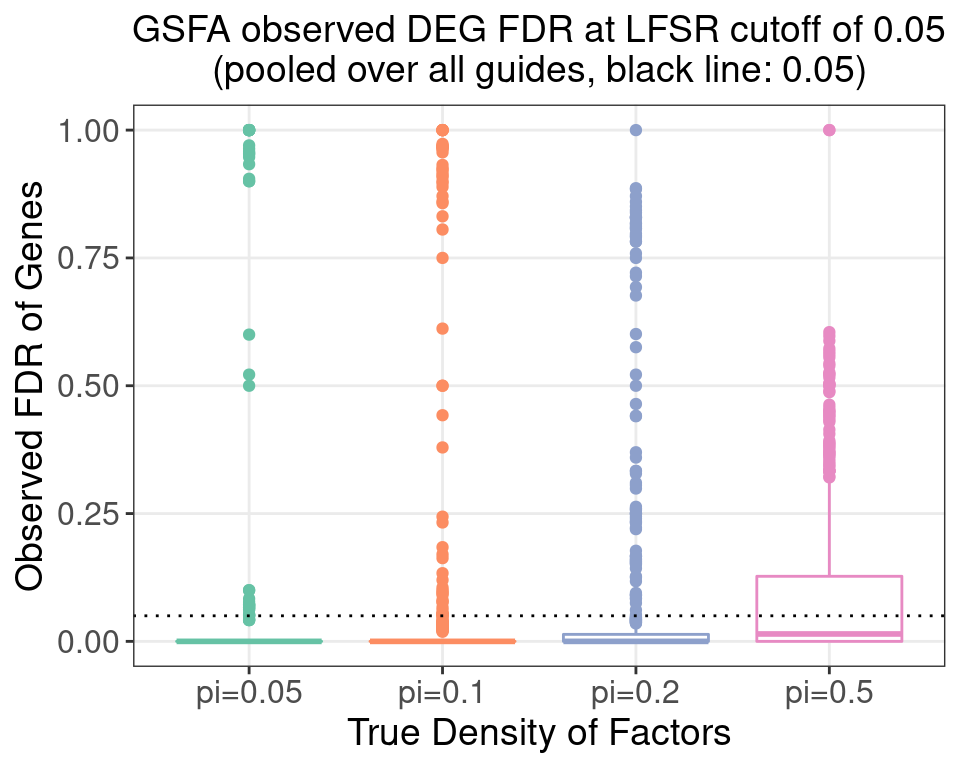
4.3.2 \(\pi\) = 0.05
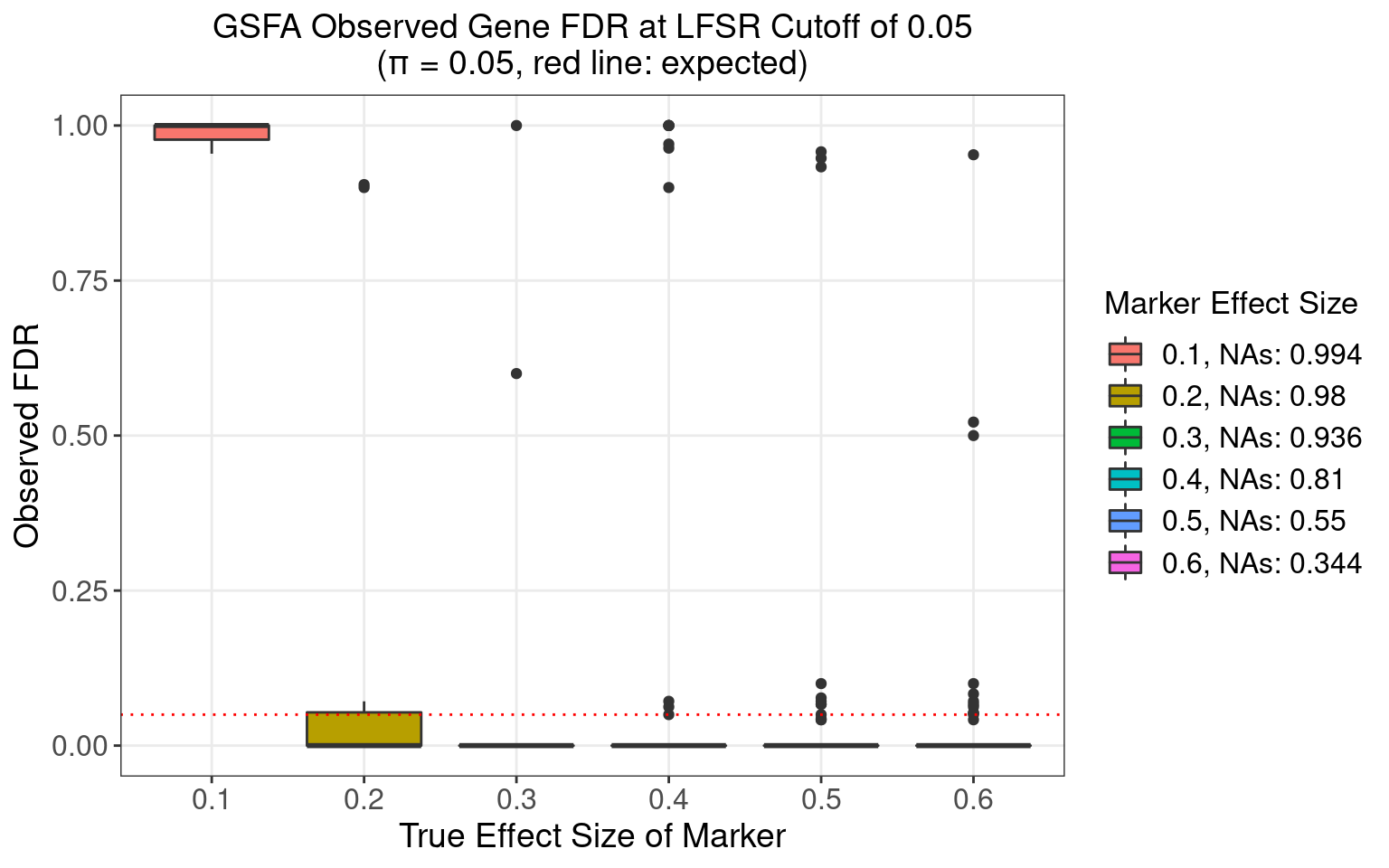
4.3.3 \(\pi\) = 0.1
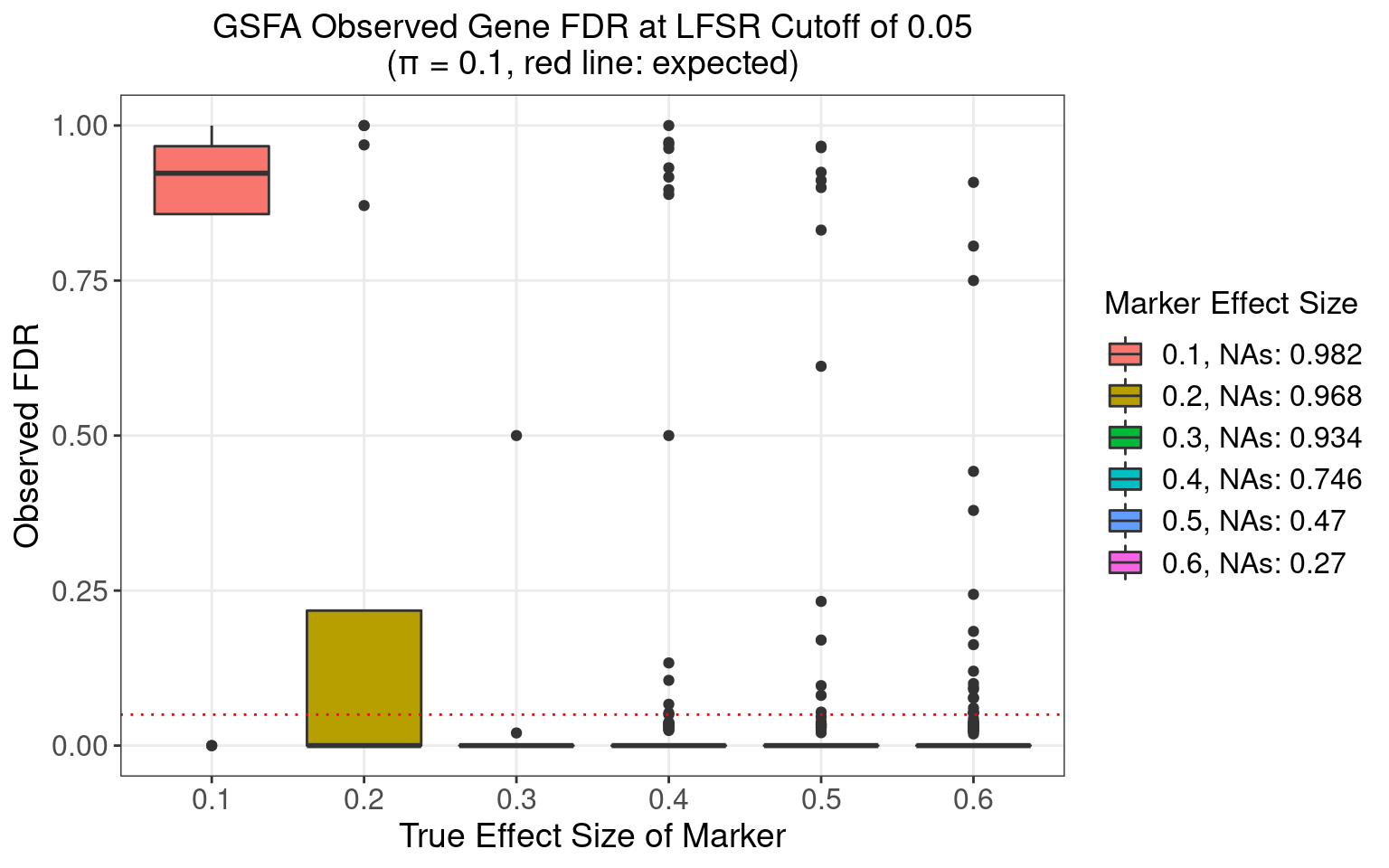
4.3.4 \(\pi\) = 0.2
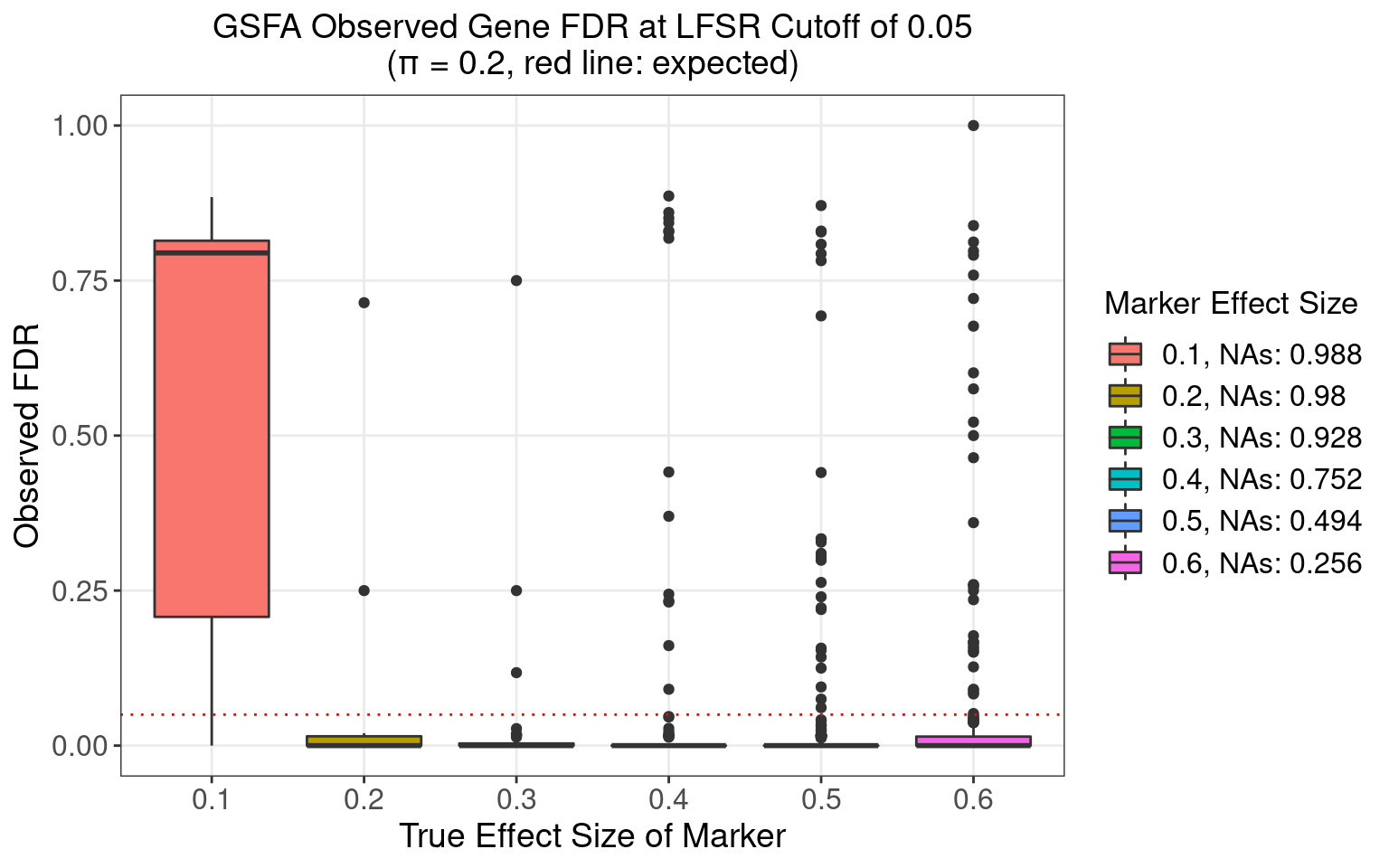
4.3.5 \(\pi\) = 0.5